 |
We are interested in developing new technologies (including novel techniques, methodologies, models, or devices) that can be used to enhance lipid research and improve human nutrition as well as diagnosis, prevention and treatment of human diseases. Over the past few years, we have made several breakthroughs in technology development. Our current projects, in collaboration with researchers in different disciplines, aim to create revolutionary technologies or products for research and clinical practice.
|
|
|
more information
Fat-1 transgenic technology (converting omega-6 to omega-3)
|
||
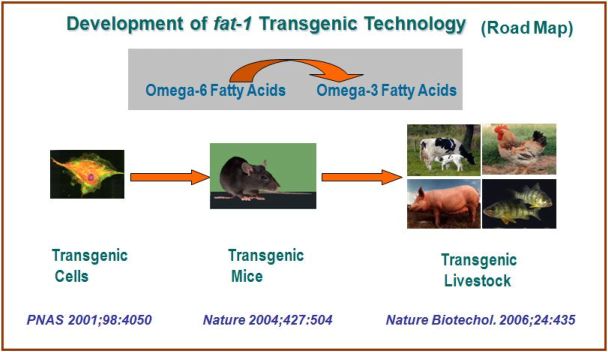 As the health benefits of long chain omega-3 fatty acids, found mainly in fish oils, have been well recognized, the demand for these fatty acids has been growing in recent years. A great effort has been made in food industry to incorporate omega-3 fatty acids into the food supply, especially meat products, which normally contain little omega-3 fatty acids but large amounts of omega-6 fatty acids. Because the essential omega-3 fatty acids cannot be de novo synthesized nor be derived from other fatty acids in mammals and other food animals, they must be obtained from the diet. Thus, the only possible way so far to enrich animal tissues with omega-3 fatty acids has been dietary supplementation (i.e. feeding animals with omega-3 fats derived from flax seed, fishmeal or other marine products), which is unsustainable because not only is it costly and time consuming, but the source of marine products is also limited and contaminated. To solve these problems, we have invented a sustainable way to provide land-based dietary sources of omega-3 fatty acids by genetically modifying animals to produce their own omega-3 fatty acids without the need of dietary supplementation. Our strategy is to convert omega-6 to omega-3 fatty acids by introducing a double bond into the omga-6 hydrocarbon chain using a gene (namely fat-1 ) encoding an omega-3 fatty acid desaturse from the roundworm C. elegans. We proved this concept in cultured cells (by adenovirus-mediated gene transfer) in 2001 (PNAS 2001;98:4050) and successfully generated the world’s first “omega-3-producing” mammal (mouse) (by the method of microinjection) in 2004 (Nature 2004;427:504) as well as the first omega-3 livestock (pig) (by nuclear transfer cloning) in 2006 (Nature Biotechnology 2006;24:435). This discovery has changed reality--- the transgenic animals are now capable of producing omega-3 from omega-6 fatty acids and have high levels of omega-3 fats in all of their organs and tissues, with no need of dietary supplementation. This technology offers a new strategy for producing omega-3 fatty acid-rich animal food products (e.g. meat, milk and eggs). This would allow people to continue to eat the foods they enjoy and still obtain the beneficial omega-3 fatty acids they need for their health without stringent dietary changes. The dependence on the limited supply and costly consumption of fish would become unnecessary. Thus, this discovery may have a great impact on both agriculture and human nutrition. We are currently collaborating with other scientists to generate "omega-3-producing" cows, chickens, and fish. We are also interested in developing other nutrients-enhanced foods. Our long-term goal is to create healthy food (especially animal food products with optimal lipid composition), through genetic engineering.
Selected Publications:
|
||
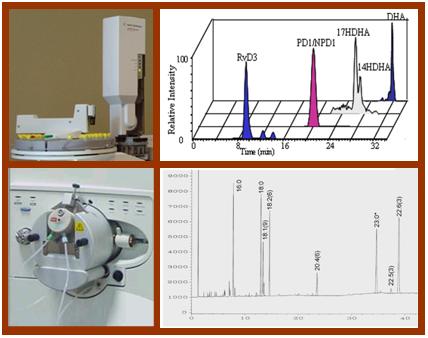
Selected Publications:
|
||
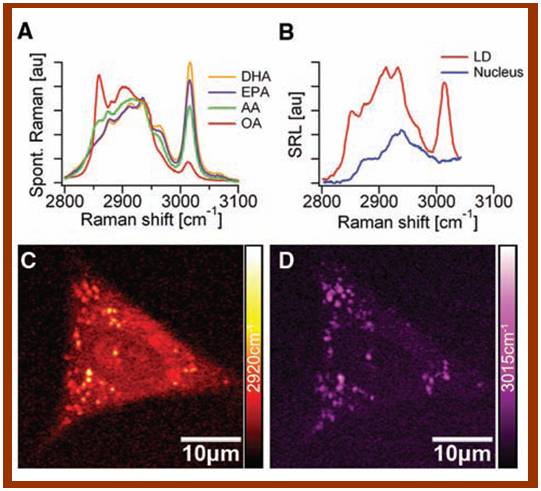
In collaboration with Prof. Sunney Xie of Harvard University, we are developing live cell and label-free imaging methods to allow direct visualization and quantitative analysis of cellular lipids. An area of special interest to us is the detection of polyunsaturated fatty acids, especially omega-3 fatty acids in living cells. Normally, fluorescent labels are used for tacking lipid molecules but their introduction can alter the original structure of lipid molecules and may have some side effects. The non-imaging analytical techniques such as gas chromatography and mass spectrometer are destructive and do not give sub-cellular resolution. We have recently developed a new multi-photon vibrational imaging technique using stimulated Raman scattering microscopy. With this technique, we are now able to observe distribution and interactions of cellular lipids such as omega-3 fatty acids in living cells with no destruction to cells and no need of labeling. This technology has a great potential as a new tool for lipid research. We are currently optimizing the technology and exploring its potential applications. We are also developing other imaging techniques, arming at mapping of the spatio-temporal dynamics of lipids and their interactions with other molecules in living cells.
Selected Publications:
|
||